Most of you did not get to attend CanoLAB 3-D in Edmonton this week, so this edition of Canola Watch will bring you the highlights. CanoLAB was a unique chance to see what real canola plants look like when they are suffering from various stresses at different growth stages. The interactive and hands-on lab was March 5 and 6 at Hole’s new Enjoy Centre in St. Albert, Alberta. The CCC and Alberta Canola Producers Commission created CanoLAB 3-D in cooperation with the University of Alberta and the Enjoy Centre.
CanoLAB had 7 stations with a different theme and a different team of experts for each. Groups of 15 growers and agronomists rotated through the stations throughout the day. The following articles will explain the key canola agronomy concepts discussed at each station.

Station 1: Weed control tips and herbicide management
Station hosts:
Neil Harker, Agriculture and Agri-Food Canada
Eric Johnson, Agriculture and Agri-Food Canada
Shawn Senko, CCC
Keith Topinka, University of Alberta
Five quick snappers from this presentation:
- Herbicide efficacy often goes down in dry conditions because most plants — weeds and crop — will develop a waxy layer and curl up their leaves to reduce moisture loss. These two survival responses also reduce herbicide uptake. (See the photos below.)
-
- If you sprayed in dry conditions and didn’t see any clear weed control after 10 or 11 days, would you spray again? In sample pots shown at this station, off-type canola volunteers sprayed 10-11 days earlier were stunted but not clearly dead. In the field, canola should advance past these sprayed volunteers and close the canopy before the volunteers recover — if they recover. A second spray would probably not provide an economic return in this case. However, for weeds like thistle or dandelion that can effectively compete with canola or weeds like cleavers where the seed in the canola sample can cause additional economic loss from downgrading, if they look like they may recover a second spray may be warranted.
-
- Imidazilonone (Odyssey, Solo, Ares, Assert) herbicide carryover damage can be worse in acid soils (pH below 6.0). Herbicide molecules will bind with soil particles, but in acid soils, the Imi herbicide molecules are more easily “washed off” the soil with a rain and can be taken up by canola plants. Other Group 2 herbicides like sulfonylureas and flucarbazone are less persistent in acidic conditions and are less likely to injure rotational crops like canola. Watch for greater persistence in those herbicides if your soil is high pH.
-
- Glyphosate applied past the 6- to 7-leaf stage of Round Ready canola will not improve yields in fields with good canola stands. Applications at flowering may actually reduce yield by causing the plant to abort flowers and pods.
-
- CleanStart tip: Apply with at least 10 gallons-per-acre carrier volume using a medium spray quality for best control. Consider adding a non-ionic surfactant or Merge if you have it. Volunteer canola is best controlled if it has no more than 2 leaves.
-
This photo shows the difference in growth between the drier tub on the left, and well watered plants on the right. Without water, crops and weeds will often put a protective waxy layer on top leaves to preserve moisture. That gives the leaves a blue appearance. Source: Keith Topinka

After withholding water for one week, top canola leaves turn blue with a waxy layer to preserve moisture. Weeds do the same thing, which hampers herbicide uptake. Note, this is a single, unreplicated comparison done indoors, however the plant appearances are typical of what we would find under drier conditions. Source: Keith Topinka
Station 2: Bertha, diamondback and their natural enemies
Station hosts:
Adam Blake, University of Alberta
Lloyd Dosdall, University of Alberta
Scott Meers, Alberta Agriculture and Rural Development
Diamondback moth adults fly into Western Canada each summer to lay eggs in canola. Within two weeks, the eggs hatch and larvae start feeding.
Two natural enemies — Diadegma and Cotesia — make the trip with them. These wasps lay eggs inside diamondback larvae. When the larvae hatch, they feed on the diamondback moth larvae from the inside out, killing them. (See the photo below.)
Bertha armyworm overwinters here and has a few natural enemies. They are an orange wasp called Banchus, which lays eggs in the bertha armyworm, and Tachinid flies, which lay eggs on the backs of bertha armyworms. Tachinid eggs hatch within minutes, and the tiny larvae bore into the bertha’s body. When these two natural parasitoids reach levels where they’re attacking 60% of the bertha population, we can expect to see bertha populations drop the following year and enter the downward side of their cycle.
Growers cannot expect natural enemies to provide enough immediate control to reduce the pest threat to canola in that year. Growers will still have to spray if economic thresholds are reached. The key is to pay attention to thresholds and spray only when necessary. This will help maintain the natural enemy numbers. (Bertha armyworm spray thresholds are pasted below.)
There is lots of insect activity in fields. Insecticide sprays stop all of it — the good and the bad.
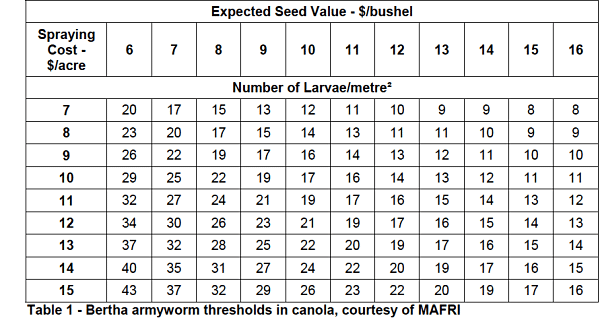
The intersection between spraying cost at the left and seed value at the top will be the number of bertha's per square metre that may warrant a spray.

A Cotesia wasp larva emerges from a diamondback moth larva. Adult Cotesia wasps lay their eggs in diamondback moth larvae, and each hatched feeds on its host, killing it. Source: Lloyd Dosdall, U of A
Station 3: Lygus and its natural enemies
Station hosts:
Jennifer Otani, Agriculture and Agri-Food Canada
Troy Prosofsky, CCC
Our current economic threshold tables for lygus in canola may be too low when applied to current canola production systems across the Prairies. The original economic threshold research was based on field studies between 1989-1993 in southern Manitoba using Westar variety and plant stands of 7-14 per square foot. The thresholds were validated in 1999-2001 with Q2 variety in Lethbridge, Beaverlodge and Dawson Creek. But that was 10+ years ago. New hybrid canola varieties grow, develop, and yield differently than Westar and Q2.
For example, if the stand is healthy and growing fast, growers are reportedly doubling or tripling thresholds. There is no data to support this, but future research needs to reexamine the thresholds with respect to our newer varieties of canola.
Even if doubling or tripling of thresholds, the scouting process depends on accurate identification of the insects netted and on proper sweep net technique.
Sweep net instructions
- Lygus sweep net monitoring should start at the late rosette stage and continue through the growing season.
-
- Sample several locations in the field. More sweeps will provide a better assessment of pest populations.
-
- Sweep-net monitoring should be done under fair weather conditions (e.g., sunny, low wind, above 15 C and between 10 a.m. and 6 p.m.) to ensure Lygus are active within the canopy. Sweep using an 180° arc into the canopy and aim to sweep the buds, flowers and pods while moving forward.
-
- Properly identify Lygus adults from similar Alfalfa plant bug adults. (See the photos below.)
-
- There are five instar or juvenile stages of Lygus nymphs. All are bright green and very mobile. Third to fifth instar nymphs will develop black spots on their backs. (See the photo below.)
-
- Identify and count third to fifth instar Lygus nymphs when sweep-net sampling at late flower and early pod stages of canola development. Nymphs plus adults should be counted and compared to the threshold tables at late flower stages because: (1) Third, fourth and fifth instar nymphs experiencing warm growing conditions can develop through an instar stage every 1-2 days. (2) Both nymphs and adults feed on canola. (3) When sweep-net sampling at mid-flowering, third, fourth and fifth instar Lygus nymphs will contribute to adult Lygus populations at the vulnerable early pod stages.
Interestingly, a small amount of lygus feeding may actually encourage the plant to produce more buds. Alberta studies found that Q2 canola plants caged with Lygus adults at bud stage produced slightly higher yields than pest-free treatments grown in the same plots. These plots received good soil moisture and average temperatures through the growing season.
Look for beneficials. Laboratory studies show that lacewing larva consumed seven Lygus nymphs every 24 hours. (See the photo below.) Damselbugs and crab spiders also consumed large numbers of lygus nymphs. Lady bugs (a.k.a. ladybird beetles) consumed low numbers of third, fourth and fifth instar Lygus nymphs. This suggests to us that these general predators (1) all play a role in suppressing Lygus in canola within the canopy, and (2) may have “feeding niches,” with each predator preferring specific nymphal instar stages as prey. These general predators exert a level of control that broad-spectrum, contact insecticides cannot.
Canola growers can conserve and protect these natural enemies by only spraying when monitoring reveals insect numbers that exceed economic thresholds, indicating that an insecticide is required to manage outbreak situations. It may be convenient to mix an insecticide with a herbicide or fungicide application, but if unnecessary these sprays may contribute to insect resistance and loss of beneficial insect populations.
A few Lygus in a field isn’t a bad thing. Sometimes they can improve canola yield but they also retain populations of these general predators.
Click here to see more...