By Rodrigo Onofre and Timothy Todd
Soybean cyst nematode (SCN) is a major problem in soybean fields throughout eastern and central Kansas (Figure 1). It is important to monitor SCN levels regularly to determine if management strategies, such as variety resistance and crop rotation, have been successful.
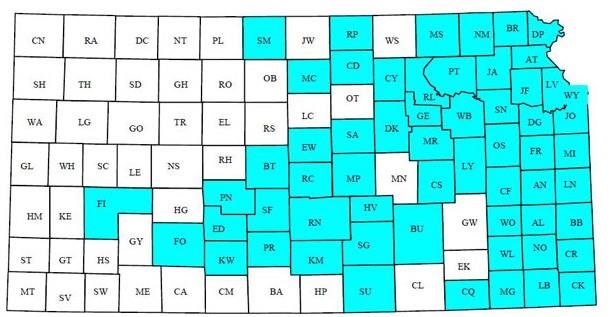
Figure 1. As of January 1, 2020, SCN was identified in 59 Kansas counties that produce >85% of Kansas soybeans. Graphic courtesy of Timothy Todd.
Immediately following harvest is the best time to check fields for SCN and start planning for next season. Confirming the presence of SCN and determining population levels is the basis for a successful integrated management program.
To make that process easier, the K-State Plant Disease Diagnostic Lab is now offering free SCN testing for Kansas producers. This program is facilitated by a grant received from the SCN Coalition. Below is some additional information about SCN and details about collecting and shipping a good sample.
To collect a SCN sample you will need:
- A soil probe (or sharpshooter spade)
- A bucket
- A labeled bag. Label should include the following information:
- Field identification (i.e. Field ID: North Farm, near Doe Creek)
- Size of the area being sampled (i.e. 20 acres)
- Crop history (i.e. soybean, corn, and soybean)
Recommended field pattern for sample collection:
If your field is fairly uniform, divide it into quadrants for your SCN sample collection. Sections of the field that have had different cropping histories or have a different soil type should be sampled separately. For each quadrant or area of the field, you will collect 10 to 20 cores to a depth of 6 to 8 inches.
It is important that when collecting soil cores you walk in a systematic pattern, such as a “Z” pattern (Figure 2). Collect a total of 10 to 20 soil cores, emptying each into the bucket after collection. All core samples should be mixed well, to account for any minor variation between cores. After mixing, collect 1 pint of soil, approximately 2 cups, in a labeled plastic bag and seal.
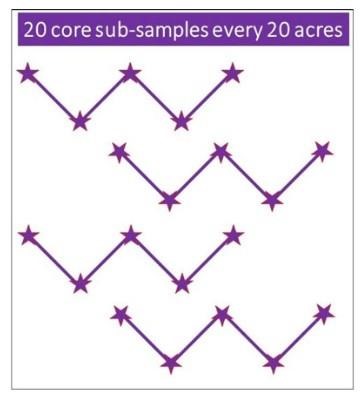
Figure 2. Example of a good sampling pattern for collecting soil to test for SCN.
When sending your samples to the diagnostic lab make sure to:
- Send overnight or as fast as possible
- Avoid leaving bags in the sun
- Send the samples to the Plant Disease Diagnostic Lab in the K-State Plant Pathology Department.
- You can find the Plant Disease Diagnostic Check sheet at https://www.plantpath.k-state.edu/extension/diagnostic-lab/documents/2021_PP_DiseaseLabChecksheet.pdf.pdf
Source : ksu.edu